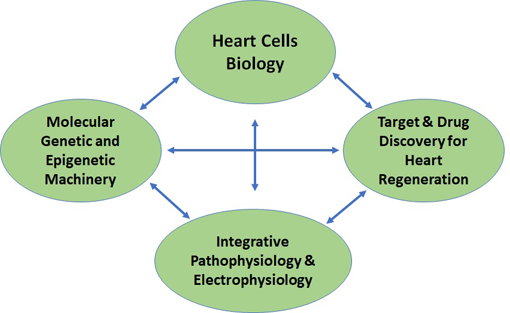
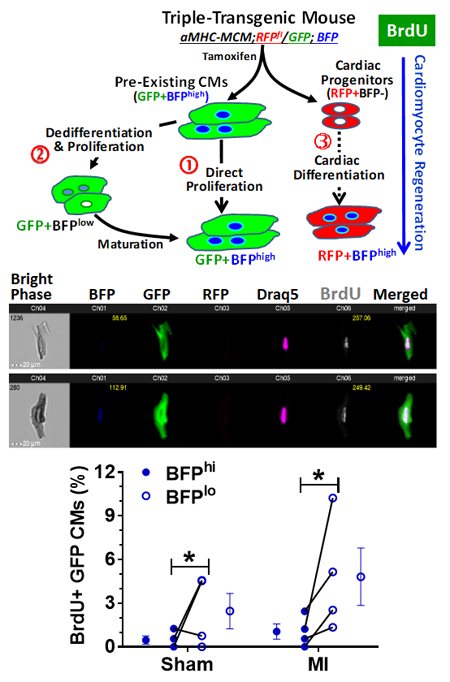
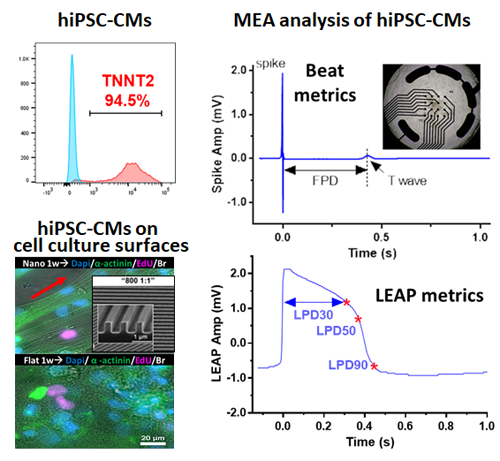
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
||||||||||||||||||||
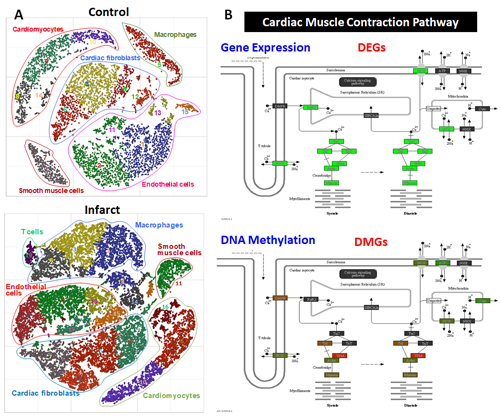
Figure 3: System cardiac genomics. A, t-SNE plot showing cell populations in normal and post-infarct triple-transgenic mouse hearts revealed by massive parallel single-nucleus RNA-seq (snRNA-seq) analysis. B, Integrative transcriptomic and DNA methylomic analysis of differentially expressed (DEGs) or methylated genes (DMGs) in dedifferentiated cardiomyocytes.
Selected publications:
- Zhang Y, Gago-Lopez N, Li N, Zhang Z, Alver N, Liu Y, Martinson AM, Mehri A, MacLellan WR. Single-cell imaging and transcriptomic analyses of endogenous cardiomyocyte dedifferentiation and cycling. (Nature) Cell Discovery, 2019; 5: 30; June 4, 2019; PMCID: PMC6547664. DOI : 10.1038/s41421-019-0095-9
- El-Nachef D, Oyama K, Wu YY, Liu Y, Zhang Y, MacLellan WR. Repressive histone methylation regulates cardiac myocyte cell cycle exit. J Mol Cell Cardiol. 2018; 121:1-12; PMID: 29800554 DOI: 10.1016/j.yjmcc.2018.05.013
- Chen X*, Chakravarty T*, Zhang Y*, Li X, Zhong JF, Wang C. Single-cell transcriptome and epigenomic reprogramming of cardiomyocyte-derived cardiac progenitor cells. (Nature) Scientific Data. 2016; 3: 160079. PMID: 27622691 (*co-first author)
- * Zhang Y, Zhong JF, Qiu H, MacLellan WR, Marban E, Wang C. Epigenomic reprogramming of adult cardiomyocyte-derived cardiac progenitor cells. (Nature) Sci. Rep. 2015; 5: 17686; doi: 10.1038/ srep17686. PubMed PMID: 26657817
- * Zhang Y, Mignone J, MacLellan WR, Cardiac Regeneration and Stem Cells. Physiol. Rev. 2015; 95: 1189-204. PubMed PMID: 26269526
- Gago-Lopez N, Awaji O, Zhang Y, Ko C, Nsair A, Liem D, Stempien-Otero A, MacLellan WR. THY-1 receptor expression differentiates cardiosphere-derived cells with divergent cardiogenic differentiation potential. Stem Cell Report, 2014;2:1-16. PMID: 24936447
- Zhang Y, Matsushita N, Eigler T, Marban. Targeted microRNA interference promotes postnatal cardiac cell cycle re-entry. J Regenerative Med. 2013;2:2. PMID: 24910852
- Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191-209. PMID: 23255322
- Li Z, Zhang C, Weiner LP, Chiou PY, Zhang Y, Zhong JF. Molecular characterization of heterogeneous mesenchymal stem cells with single-cell transcriptomes. Biotechnol. Adv. 2013;31(2):312-7. PMID: 23266308 )
- Barth AS*, Zhang Y*, Li TS, Smith RR, Chimenti I, Terrovitis J, Davis D, Kizana E, Ho A, O'Rourke B, Wolff A, Gerstenblith G, Marban E. Functional impairment of human resident cardiac stem cells contributes to trastuzumab cardiotoxicity. Stem Cells Transl Med, 2012;1(4):289-97.. PMID: 23197808 (* equal first-author)
- Li TS, Cheng K, Malliaras K, Matsushita N, Sun B, Marban L, Zhang Y, Marban E. Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovascular Res., 2011;89: 157-165. PMID: 20675298
- Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham RM, Wang C, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One 2010; 5: e12559. PMID: 20838637
View a complete list of Publications in MyBibliography: Click here
OPPORTUNITIES
Positions
Our laboratory and institution provide a broad spectrum of learning opportunities for undergraduate students, graduate students and postdoctoral fellows in the following areas: Cellular Molecular Biology, Heart Diseases, Animal models, Stem Cell Research and Regenerative Medicine, Genomics and Epigenetics, Electrophysiology, and Bioengineering.
Our lab can only accept graduate students who have already been admitted to the University of Hawaii Manoa (UHM) graduate programs. Candidates are encouraged to apply through the Department of Anatomy, Biochemistry and Physiology, or other departments at the UHM. For undergraduate students, independent study for potential credit and completion of an undergraduate thesis are highly encouraged. Outstanding and motivated undergraduates with necessary knowledge and skills from all departments who can commit at least 12 hours per week and plan to stay with our lab during the academic year, can contact Dr. Zhang by e-mail and submit a CV and statement of interest as a single PDF.
Research Scientist and Post-docs interested in the opportunities are encouraged to contact Dr. Zhang directly by sending the following documents in a single PDF file via e-mail: 1) a cover letter outlining their research interests and career plans; 2) a CV including three references and their contact information; 3) any selected publications.
- Zhang Y, Gago-Lopez N, Li N, Zhang Z, Alver N, Liu Y, Martinson AM, Mehri A, MacLellan WR. Single-cell imaging and transcriptomic analyses of endogenous cardiomyocyte dedifferentiation and cycling. (Nature) Cell Discovery, 2019; 5: 30; June 4, 2019; PMCID: PMC6547664. DOI : 10.1038/s41421-019-0095-9
- El-Nachef D, Oyama K, Wu YY, Liu Y, Zhang Y, MacLellan WR. Repressive histone methylation regulates cardiac myocyte cell cycle exit. J Mol Cell Cardiol. 2018; 121:1-12; PMID: 29800554 DOI: 10.1016/j.yjmcc.2018.05.013
- Chen X*, Chakravarty T*, Zhang Y*, Li X, Zhong JF, Wang C. Single-cell transcriptome and epigenomic reprogramming of cardiomyocyte-derived cardiac progenitor cells. (Nature) Scientific Data. 2016; 3: 160079. PMID: 27622691 (*co-first author)
- * Zhang Y, Zhong JF, Qiu H, MacLellan WR, Marban E, Wang C. Epigenomic reprogramming of adult cardiomyocyte-derived cardiac progenitor cells. (Nature) Sci. Rep. 2015; 5: 17686; doi: 10.1038/ srep17686. PubMed PMID: 26657817
- * Zhang Y, Mignone J, MacLellan WR, Cardiac Regeneration and Stem Cells. Physiol. Rev. 2015; 95: 1189-204. PubMed PMID: 26269526
- Gago-Lopez N, Awaji O, Zhang Y, Ko C, Nsair A, Liem D, Stempien-Otero A, MacLellan WR. THY-1 receptor expression differentiates cardiosphere-derived cells with divergent cardiogenic differentiation potential. Stem Cell Report, 2014;2:1-16. PMID: 24936447
- Zhang Y, Matsushita N, Eigler T, Marban. Targeted microRNA interference promotes postnatal cardiac cell cycle re-entry. J Regenerative Med. 2013;2:2. PMID: 24910852
- Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191-209. PMID: 23255322
- Li Z, Zhang C, Weiner LP, Chiou PY, Zhang Y, Zhong JF. Molecular characterization of heterogeneous mesenchymal stem cells with single-cell transcriptomes. Biotechnol. Adv. 2013;31(2):312-7. PMID: 23266308 )
- Barth AS*, Zhang Y*, Li TS, Smith RR, Chimenti I, Terrovitis J, Davis D, Kizana E, Ho A, O'Rourke B, Wolff A, Gerstenblith G, Marban E. Functional impairment of human resident cardiac stem cells contributes to trastuzumab cardiotoxicity. Stem Cells Transl Med, 2012;1(4):289-97.. PMID: 23197808 (* equal first-author)
- Li TS, Cheng K, Malliaras K, Matsushita N, Sun B, Marban L, Zhang Y, Marban E. Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovascular Res., 2011;89: 157-165. PMID: 20675298
- Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham RM, Wang C, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One 2010; 5: e12559. PMID: 20838637
View a complete list of Publications in MyBibliography: Click here
Positions
Our laboratory and institution provide a broad spectrum of learning opportunities for undergraduate students, graduate students and postdoctoral fellows in the following areas: Cellular Molecular Biology, Heart Diseases, Animal models, Stem Cell Research and Regenerative Medicine, Genomics and Epigenetics, Electrophysiology, and Bioengineering.
Our lab can only accept graduate students who have already been admitted to the University of Hawaii Manoa (UHM) graduate programs. Candidates are encouraged to apply through the Department of Anatomy, Biochemistry and Physiology, or other departments at the UHM. For undergraduate students, independent study for potential credit and completion of an undergraduate thesis are highly encouraged. Outstanding and motivated undergraduates with necessary knowledge and skills from all departments who can commit at least 12 hours per week and plan to stay with our lab during the academic year, can contact Dr. Zhang by e-mail and submit a CV and statement of interest as a single PDF.
Research Scientist and Post-docs interested in the opportunities are encouraged to contact Dr. Zhang directly by sending the following documents in a single PDF file via e-mail: 1) a cover letter outlining their research interests and career plans; 2) a CV including three references and their contact information; 3) any selected publications.
 |