|
|

Michelle Tallquist
|
 |
| Affiliation: |
JABSOM, Center for Cardiovascular Research |
| Position: |
Associate Professor |
| Degree: |
PhD (Immunology, Mayo Clinic and Foundation) |
| Phone: |
(808) 692 1579 |
| Fax: |
(808) 692 1973 |
| Email: |
michelle.tallquist@hawaii.edu |
| Address: |
BSB 311E, 651 Ilalo Street, Honolulu HI, 96813 |
|
Research projects:
Development and disease of the cardiovascular system with a special emphasis on fibroblasts
Description of research:
The ability to repair damaged organs using stem cells is a potential mechanism for treatment of certain diseases, but one of the factors that reduces this possibility is the presence of excess extracellular matrix (ECM) within the tissue. The presence of this ECM is called fibrosis, and it is common in many different diseased organs including the heart, liver, kidney, and lung. One area of research in our lab is to discover genes that are required for the embryonic formation of the resident cells that produce this matrix. This cell is called the fibroblast. Our current focus is on the fibroblast populations present within the heart. We believe that by understanding the normal development of these cells, we may discover important signaling pathways that are involved during the expansion of these cells during disease. In the pursuit of this goal, we have identified two genes that are required for cardiac fibroblast formation and are currently determining additional genes involved in the development of this cell population. A second area of investigation is the role that vasculature plays in mesenchymal stem cell biology. Using mice that have abnormal vasculature, we are defining the cellular components necessary for mesenchymal stem cell formation and function. In the future we will use clonal analysis to define where mesenchymal stem cells arise during development as well as during regeneration.
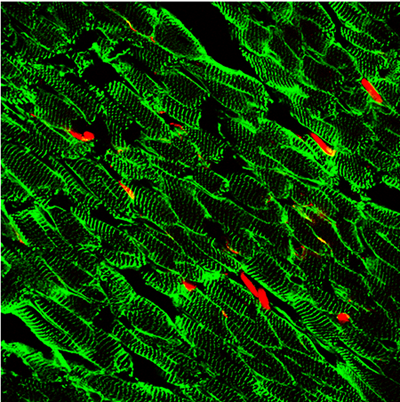
Figure 1: A novel tagging system for the identification of the cardiac fibroblast.
Selected publications:
- Krautler N, Kana V, Kranich J, Tian Y, Perera D, Lemm D, Schwarz P, Armulik A, Browning J, Tallquist M D, Buch T, Oliveira-Martins J. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 2012;150:194-206.
- Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012;485:599-604
- Chhabra A, Lechner AJ, Ueno M, Acharya A, Van Handel B, Wang Y, Iruela-Arispe ML, Tallquist MD, Mikkola HK. Trophoblasts regulate the placental hematopoietic niche through pdgf-b signaling. Dev Cell. 2012;22:651-659
- Baek ST, Tallquist MD. Nf1 limits epicardial derivative expansion by regulating epithelial to mesenchymal transition and proliferation. Development. 2012;139:2040-2049
- Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bhlh transcription factor tcf21 is required for lineage-specific emt of cardiac fibroblast progenitors. Development. 2012
- Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require pdgf receptor signaling. Circ Res. 2011;108:e15-26
- Caglayan E, Vantler M, Leppanen O, Gerhardt F, Mustafov L, Ten Freyhaus H, Kappert K, Odenthal M, Zimmermann WH, Tallquist MD, Rosenkranz S. Disruption of platelet-derived growth factor-dependent phosphatidylinositol 3-kinase and phospholipase cgamma 1 activity abolishes vascular smooth muscle cell proliferation and migration and attenuates neointima formation in vivo. J Am Coll Cardiol. 2011;57:2527-2538
- Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. Efficient inducible cre-mediated recombination in tcf21 cell lineages in the heart and kidney. Genesis. 2011;49:870-877
- Wu M, Smith CL, Hall JA, Lee I, Luby-Phelps K, Tallquist MD. Epicardial spindle orientation controls cell entry into the myocardium. Dev Cell. 2010;19:114-125
- Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. Lrp1 regulates architecture of the vascular wall by controlling pdgfrbeta-dependent phosphatidylinositol 3-kinase activation. PLoS One. 2009;4:e6922
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625-636
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583-586
- Pickett EA, Olsen GS, Tallquist MD. Disruption of pdgfralpha-initiated pi3k activation and migration of somite derivatives leads to spina bifida. Development. 2008;135:589-598
- Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res. 2008;103:1393-1401
- French WJ, Creemers EE, Tallquist MD. Platelet-derived growth factor receptors direct vascular development independent of vascular smooth muscle cell function. Mol Cell Biol. 2008;28:5646-5657
- Richarte AM, Mead HB, Tallquist MD. Cooperation between the pdgf receptors in cardiac neural crest cell migration. Dev Biol. 2007;306:785-796
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044-1051
- Tallquist MD, Soriano P. Cell autonomous requirement for pdgfralpha in populations of cranial and cardiac neural crest cells. Development. 2003;130:507-518
- Tallquist MD, French WJ, Soriano P. Additive effects of pdgf receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52
- Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the dab2 adaptor protein in embryonic development and kidney transport. Embo J. 2002;21:1555-1564
|
|



