|
|

Yusuke Marikawa
|
 |
| Affiliation: |
School of Medicine, Institute for Biogenesis Research |
| Position: |
Associate Professor |
| Degree: |
PhD (Kyoto University, Japan) |
| Phone: |
(808) 692 1411 |
| Fax: |
(808) 692 1962 |
| Email: |
marikawa@hawaii.edu |
| Address: |
651 Ilalo St., BSB 163A, Honolulu, HI 96813 |
|
Research projects:
Axis Specification and Germ Layer Formation in Mammalian Embryos.
Differentiation and Morphogenesis in Pluripotent Stem Cells.
Stem Cell-Based Detection of Teratogens.
Description of research:
How can a single cell, like the fertilized egg, transform into a complex architecture, like our body? With respect to this biggest question of Developmental Biology, my lab is currently conducting research projects under the following two main themes:
"Axis Specification and Germ Layer Formation in Mammalian Embryos"
Soon after implantation, the embryo of human or mouse is a single layer of epithelial tissue, called the epiblast. Later, one edge of the epiblast forms the structure called the primitive streak, from which cells migrate away to form new tissue layers. This event, i.e., the formation of the primary germ layers (ectoderm, mesoderm, and endoderm), is the first step towards the generation of complex body patterns. Furthermore, the primitive streak formation is the first morphological landmark of body axis specification, as it establishes the caudal end of the future body.
Activation of Wnt/beta-catenin signaling, an evolutionary conserved signal transduction machinery, is the key to the formation of the primitive streak in the epiblast. The specific questions that are addressed in my current projects are:
1) How Wnt/beta-catenin signaling is activated in a localized region of the epiblast?
2) What genes are turned on by Wnt/beta-catenin signaling in the primitive streak?
3) How can cells in the primitive streak transform from epithelial into migratory state?
"Differentiation and Morphogenesis in Pluripotent Stem Cells"
Embryonic stem (ES) cells and induced pluripotent stem (iPS) cells can be maintained and propagated in a culture dish as undifferentiated cell populations, which are very similar to early embryonic cells before germ layer formation. Because studies of mammalian embryos are often hindered by the unique reproductive mode of mammals (i.e., embryos normally develop in the uterus), pluripotent stem cells, like ES and iPS cells, can serve as great in vitro models, which can be experimentally manipulated more easily. Furthermore, these pluripotent stem cells offer tremendous promise for the future regenerative medicine, as they can be used to derive various types of functional tissues in vitro, and those stem cell-derived tissues may be used for tissue replacement therapy and drug screening.
The goal of my lab is to elucidate molecular and cellular mechanisms of differentiation (e.g., of mesoderm and endoderm tissues) and morphogenesis (e.g., of tissue elongation along the body axis) using mouse and human pluripotent stem cells.
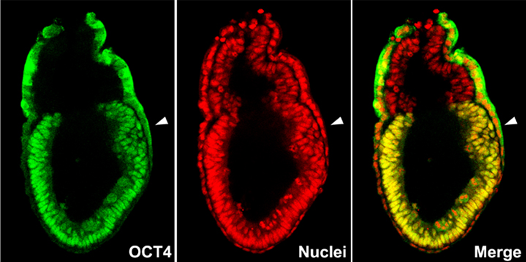
Figure1: Confocal microscopy image of mouse E6.5 stage embryo, showing the distribution of pluripotency regulator OCT4 protein (green) and all the nuclei (red). The epiblast shows nuclear localization of OCT4 protein. Arrowhead indicates the site of the primitive streak formation.

Figure 2: in vitro axial elongation morphogenesis in aggregated P19 embryonal carcinoma cells. Scale bar = 1 mm.
Recent publications:
- Yuan CJ, Marikawa Y. (2017) Developmental toxicity assessment of common excipients using a stem cell-based in vitro morphogenesis model. Food and Chemical Toxicology. 109:376-385.
- Warkus EL, Marikawa Y. (2017) Exposure-based validation of an in vitro gastrulation model for developmental toxicity sssays. Toxicological Sciences. 157:235-245.
- Li AS, Marikawa Y. (2016) Adverse effect of valproic acid on an in vitro gastrulation model entails activation of retinoic acid signaling. Reproductive Toxicology. 66:68-83.
- Warkus EL, Yuen AA, Lau CG, Marikawa Y.Use of in vitro morphogenesis of mouse embryoid bodies to assess developmental toxicity of therapeutic drugs contraindicated in pregnancy. Toxicological Sciences. 149:15-30.
- Li AS, Marikawa Y. (2015) An in vitro gastrulation model recapitulates the morphogenetic impact of pharmacological inhibitors of developmental signaling pathways. Molecular Reproduction and Development. 82:1015-1036.
- Lau CG, Marikawa Y. (2014) Morphology-based mammalian stem cell tests reveal potential developmental toxicity of donepezil. Molecular Reproduction and Development. 81:994-1008.
- Jaremko KL, Marikawa Y. (2013) Regulation of Developmental Competence and Commitment towards the Definitive Endoderm Lineage in Human Embryonic Stem Cells. Stem Cell Research. 10:489-502.
- Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K, Ohno S, Marikawa Y, Nakao K, Shimono A, Sasaki H. (2013) Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Current Biology 23:1181-1194.
- Tamura AN, Huang TT, Marikawa Y. (2013) Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes. Biology of Reproduction 89:112
- Tamashiro DA, Alarcon VB, Marikawa Y. (2012) Nkx1-2 is a transcriptional repressor and is essential for the activation of Brachyury in P19 mouse embryonal carcinoma cell. Differentiation. 83:282-292.
- Marikawa Y, Alarcon VB. (2012) Creation of trophectoderm, the first epithelium, in mouse preimplantation development. Results and Problems of Cell Differentiation 55:165-184.
- Complete List of Published Work in MyBibliography: Click here
Sponsor websites:
|
|



