|
|

Ben Fogelgren
|
 |
| Affiliation: |
School of Medicine, Department of Anatomy, Biochemistry, and Physiology |
| Position: |
Assistant Professor |
| Degree: |
PhD (University of Hawaii) |
| Phone: |
808-692-1420 |
| Fax: |
808-692-1951 |
| Email: |
fogelgre@hawaii.edu |
| Address: |
651 Ilalo St., BSB 110, Honolulu, HI 96813 |
|
Current Research Projects:
Molecular mechanisms of primary cilia assembly and signaling
Genetic regulation of kidney development, physiology, and disease.
Description of research:
Our research is focused on the molecular and genetic causes of abnormal kidney development, as well as the novel causes and treatments of adult renal diseases such as polycystic kidney disease and acute renal injury. The same molecular signals and cellular morphogenesis patterns that we study in the kidney can also give us novel insights into development of other tissues, as well as teach us how these processes can be disrupted in a large variety of diseases.
Cysts and tubules are primary building blocks of the kidney, and defects in cystogenesis or tubulogenesis during kidney development can result in a spectrum of pediatric and adult renal diseases. Relevant to our research, during kidney development, disruption in the formation of de novo epithelial cysts from pools of mesenchymal stem cells lead to various forms of congenital abnormalities such as kidney hypoplasia, dysplasia, or agenesis. On the other hand, following nephrogenesis, when the inhibitory signals that halt excessive cyst formation are disturbed, forms of renal disease such as polycystic kidney disease (PKD) can occur. Autosomal dominant PKD (ADPKD), which affects approximately 500,000 Americans and currently has no approved treatment, is the most common potentially lethal genetic disease. Every variation of human PKD has been attributed to mutations in genes important to the assembly or function of the primary cilium, a thin rod-like organelle on renal tubular epithelial cells which projects into the tubular lumen (See Figure 1).
Primary cilia have been found on the surface of most growth-arrested or differentiated mammalians cells, including a large variety of epithelial cells, endothelial cells, connective-tissue and muscle cells, as well as neurons and even embryonic stem cells. Although the presence of primary cilia has been noted for over a hundred years, its biological function was a mystery. Due to an exploding volume of research in just the last decade, it is now believed the primary cilium acts as a "sensory antenna" to relay signals to the cell based on the extracellular environment. These can include sensory clues such as mechanical stimulation (bending or rotation), chemosensation (detection of growth factors, morphogens, etc.), osmolarity, light, temperature, and even gravitational pull. It is thought that primary cilia along the renal tubules are responsible for detecting fluid composition and flow dynamics, and when defective, the cells misinterpret this as a signal to dedifferentiate and proliferate, resulting in the formation of large pathogenic renal cysts. However, defects in primary cilia can affect other tissues as well, and have resulted in a new classification of human disorders termed "ciliopathies".
Currently, we have research projects focused both on the molecular mechanisms of de novo cyst formation, and on primary cilia assembly and signaling. We have found both of these cellular activities depend on the exocyst complex, a highly conserved eight-protein complex (see Figure 2), which is involved in targeted secretory vesicle transport. We are working hard to discover how cells regulate the exocyst subunits in order to direct polarized trafficking to specific locales such as primary cilia and the lumens of growing epithelial cysts. These discoveries may lay the groundwork for novel therapies for adult and pediatric renal diseases, and expand our knowledge of some of the basic cellular and molecular mechanisms by which the human body controls its development.
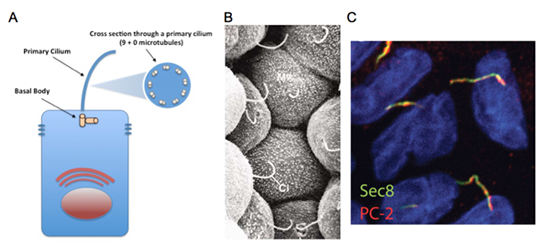
Figure 1: The primary cilium, found on almost all cell types, is a single rod-like organelle that extends out into the extracellular environment to relay information back to the cell. A. In polarized epithelial cells, the primary cilium extends out from the apical surface into the luminal space. All primary cilia are non-motile, comprised of 9 microtubule doublets lacking a central pair, extending from a basal body. B. Scanning electronic micrograph of the lumen-facing side of renal tubular epithelial cells displaying long primary cilia (from Kessel and Kardon, Tissues And Organs, 1979). C. Immunostaining of exocyst subunit Sec8 (shown in red) and polycystin-2 (the protein encoded by Pkd2 gene, shown in green) along primary cilia in cultured renal tubular epithelial cells.
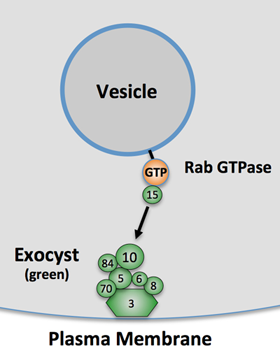
Figure 2: The exocyst is a highly-conserved complex that guides intracellular vesicles to specific sites of exocytosis (figure adapted from Lipschutz and Mostov, 2002), and is composed of eight proteins (Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Sec84). Regulation of the exocyst subunits is a method by which the cell can control both the timing and subcellular location of integral protein (and membrane) delivery to the cell's plasma membrane. For example, a subunit of the exocyst is phosphorylated in response to insulin signaling in adipocytes, which induces exocytosis of vesicles carrying the Glut4 transporter to allow intake of glucose (Inoue et al., Nature, 2002).
Selected publications:
- Polgar N, Lee AJ, Lui VH, Napoli JA, Fogelgren B. The exocyst gene Sec10 regulates renal epithelial monolayer homeostasis and apoptotic sensitivity. Am J Physiol Cell Physiol. 2015 Aug 1;309(3):C190-201. text of link
- Fogelgren B, Polgar N, Lui VH, Lee AJ, Tamashiro KK, Napoli JA, Walton CB, Zuo X, Lipschutz JH. Urothelial Defects from Targeted Inactivation of Exocyst Sec10 in Mice Cause Ureteropelvic Junction Obstructions. PLoS One. 2015 Jun 5;10(6):e0129346.
- Fogelgren B, Zuo X, Buonato JM, Vasilyev A, Baek JI, Choi SY, Chacon-Heszele MF, Palmyre A, Polgar N, Drummond I, Park KM, Lazzara MJ, Lipschutz JH. Exocyst Sec10 protects renal tubule cells from injury by EGFR/MAPK activation and effects on endocytosis. Am J Physiol Renal Physiol. 2014 Dec 15;307(12):F1334-41.
- Choi SY, Fogelgren B, Zuo X, Huang L, McKenna S, Lingappa VR, Lipschutz JH. Exocyst Sec10 is involved in basolateral protein translation and translocation in the endoplasmic reticulum. Nephron Exp Nephrol. 2012;120(4):e134-40.
- Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, and Lipschutz JH. Exocyst Sec10 interacts with polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genetics, 7(4): e1001361, doi:10.1371/journal.pgen.1001361, 2011.
- Chung DC, Fogelgren B, Park KM, Heidenberg J, Zuo XF, Huang L, Bennett J, and Lipschutz JH. Adeno-associated virus (AAV)-mediated gene transfer to renal tubule cells via a retrograde ureteral approach. Nephron EXTRA, 1: 217-223, 2011.
- Somponpun J, Wong B, Kuroyama M, Hynd T, Fogelgren B, and Lozanoff S. Osmoregulatory defect associated with hypodysplastic kidney in 3H1 Brachyrrhine mice. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 301(3): R682-R689, 2011.
- Zuo XF, Fogelgren B, and Lipschutz JH. The small GTPase cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. Journal of Biological Chemistry, 286(25): 22469-22477, 2011.
- Park KM, Fogelgren B, Zuo X, Kim J, Chung DC, Lipschutz JH. Exocyst Sec10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. American Journal of Physiology: Renal Physiology, 298(3): F818-F826, 2010.
- Fogelgren B, Yang S, Sharp IC, Huckstep OJ, Ma W, Somponpun J, Carlson EC, Uyehara C and Lozanoff S. Deficiency in Six2 during prenatal development is associated with reduced nephron number, chronic renal failure, and hypertension in Br/+ adult mice. American Journal of Physiology: Renal Physiology, 296(5): F1166-F1178, 2009.
- Fogelgren B, Kuroyama MC, McBratney-Owen B, Spence AA, Malahn LE, Anawati MK, Cabatbat C, Alarcon VB, Marikawa Y and Lozanoff S. Misexpression of Six2 is associated with heritable frontonasal dysplasia and renal hypoplasia in 3H1 Br mice. Developmental Dynamics, 237(7):1767-79, 2008.
- Polgar N, Fogelgren B, Shipley JM and Csiszar K. Lysyl oxidase interacts with hormone placental lactogen and synergistically promotes breast epithelial cell proliferation and migration. Journal of Biological Chemistry, 170(2): 578-589, 2007.
- Cao T, Racz P, Szauter KM, Groma G, Fogelgren B, Pankotai E, He QP and Csiszar K. Mutation in Mpzl3, a novel gene encoding a predicted adhesion protein, in the Rough Coat (rc) mice with severe skin and hair abnormalities. Journal of Investigative Dermatology, 127(6): 1375-86, 2007.
- Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SF, Csiszar K, Hendrix MJ and Kirschmann DA. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Research, 65(24): 11429-36, 2005.
- Fogelgren B, Polgar N, Szauter KM, Ujfaludi Z, Laczko R, Fong KS and Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. Journal of Biological Chemistry, 280(26): 24690-97, 2005.
|
|



